New drug pricing report showcases highs, lows, distorted incentives, and brokenness of Medicare Part D
We’ll try and be brief … this time
As Americans, we love a good sequel, prequel, or re-make. As diehard Star Wars fans, we have particularly strong feelings about each (Phantom Menace was a good movie; there we said it). As drug pricing researchers though, it can be a little disheartening when we see the same story repeated over and over again – especially when the story stinks.
In the United States, the fairy tale is supposed to work like this: Evil chronic conditions feast on the innocent souls of the populace, wreaking havoc on the pocketbooks and quality of life of the afflicted. At the victim’s peak moment of peril, in rides the heroic newly-approved brand therapy that vanquishes the rotten scourge into oblivion. But there’s a catch. That miraculous pharmaceutical protagonist comes with a hefty branded cost. But it’s a cost we’re willing to pay, because we know at some point towards the end of the story, the citizenry stumbles upon the hidden treasure of generic competition, and everyone lives happily ever after.
But alas, the story doesn’t always work out the way it’s supposed to. In fact, the primary antagonist that is dangerous, expensive disease often has a gang of fellow baddies that can further complicate the affordable medication’s “hero’s journey.” Patent thickets, product hops, rebate walls, inflated supply chain prices, and a slew of other seedy rabble far-too-often compromise the happy ending we expect. And unfortunately for all of us, the tragic and regrettable tale repeats itself far more than it should.
Today, after months of research and investigation, we are launching some rotten tomatoes at another one of our drug channel’s biggest stinkers. But unlike horrible flops like Caddyshack II or Police Academy IV, the sin of this bomb isn’t just its lack of humor – it’s the horrible disregard for the health and well-being of our nation’s seniors, specifically those battling multiple sclerosis. And while we can quickly toss those bad flicks into the trash bin of film history and forget about them, these stories of drug pricing woe linger and fester.
And as you’ll see in today’s newly released 46brooklyn drug pricing research report, until action is taken to re-align or re-set the incentives within our nation’s prevailing pharmacy benefits designs, we will likely continue with these obnoxious holiday re-releases year after year after year. So join us, if you dare, as we share the tired, old story of how broken Medicare’s prescription drug coverage actually is.
The Original - The Rise of the ‘Zombie Brands’ [46brooklyn’s Copaxone Report]
Two years ago, we put on our hardhats and went spelunking into the cavernous depths of the byzantine Medicare Part D plan cost share world to better understand and illustrate how the incentives created by the program’s design were bewilderingly injecting life into multi-source brands (i.e., “zombie brands”) and leading to warped, inflated pricing (and poor uptake) of their generic equivalents. We ventured down into these depths using Teva’s Copaxone as our case study, a blockbuster drug that treats certain types of multiple sclerosis (MS), and is no stranger to drama when it comes to pricing issues and patent games.
But the ironic story of how the federal government’s design of Part D (specifically its design of the coverage gap) backfired on Medicare and its seniors, protecting Copaxone from generic competition, simply had not been told. So, we wrote that story – complete with all the math that implicated the Part D program for its culpability in this scheme. But since then nothing has changed. Meaning that now, two years later, history has repeated itself for the same MS community that had to bear the brunt of the Part D program’s structural failure with Copaxone.
Be warned there are spoilers ahead.
The sequel - Wreck-fidera
Tecfidera, another blockbuster MS treatment – and Biogen’s highest revenue drug in 2019 – went generic in late-2020 after Mylan prevailed in their challenges against Biogen’s patents. The loss of patent exclusivity was actually just held up in court yesterday, so yay to our generic drug pricing hero.
Within months of the favorable ruling for the generic manufacturers in 2020, more than ten generic drug makers brought competing versions of dimethyl fumarate to market with “deeply discounted prices to Tecfidera.” Mind you, those are not our words. Those are Biogen’s words, which they provided in their latest quarterly financial report. Biogen went on to state that, “generic competition for TECFIDERA has significantly reduced our TECFIDERA revenue and is expected to have a substantial negative impact on our TECFIDERA revenue for as long as there is generic competition.” And while Biogen may have launched Vumerity to mitigate this (which in our view is a prototypical example of a product hop, if for no other reason than the fact that Biogen’s Tecfidera.com literally “website hops” and re-directs to a pitch for Vumerity), this bad news for Biogen must be good news for patients and plan sponsors, correct?
Perhaps the better question is, would we be writing this report if it was that simple?
If generic prices are “deeply discounted,” those discounts should be making it back to the payer, and ultimately, the patient … right? As you likely have guessed by the sarcastic way we phrased this rhetorical question, they are not.
As you will see in today’s newly released report, the savings are instead getting stuck in the cluttered, inefficient, junk-food-laden intestine that is the U.S. drug supply chain rather than cleanly and quickly passing out the other side to the patient.
But the story of Tecfidera is not simply part two of our Copaxone story, except this time with toilet metaphors. In our view, Tecfidera’s story is even more frustrating than Copaxone’s, because in Tecfidera’s case, the efficient, high-fiber generic marketplace actually worked very quickly and effectively, with aggressive competition piledriving prices down to where you can find a 60-count bottle of the generic equivalent today (roughly one year post generic launch) for a 99%+ discount to the brand’s list price.
That bears repeating: The generic’s market-competitive acquisition cost for generic Tecfidera is 99%+ off the brand’s list price as of November 2021.
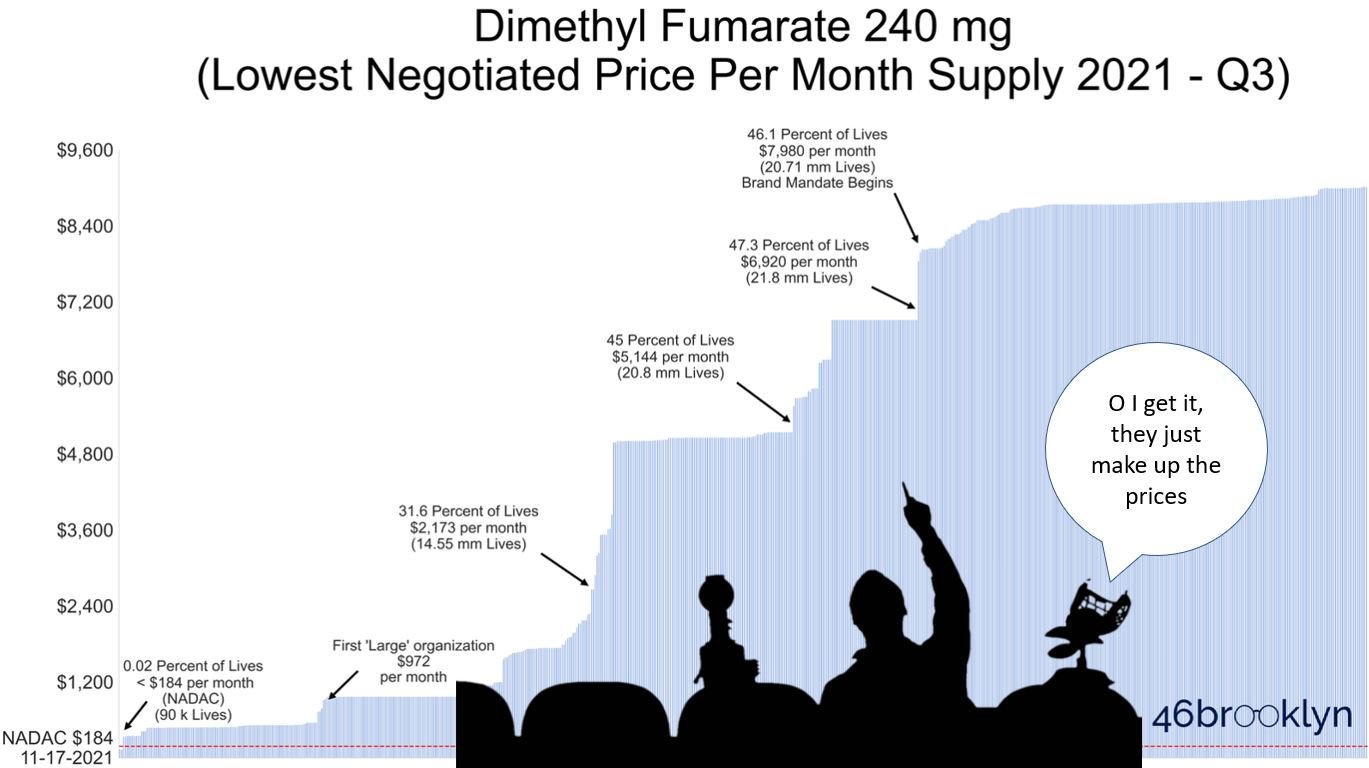
So then why did we find that in Q3 2021, Medicare Part D plans covering the majority of U.S. seniors didn’t even make the generic equivalent available to their members; instead only offering them brand-name Tecfidera? (See our trailer image below)
And then when the generic was made available to seniors, it was largely done so at “negotiated prices” that far exceeded the lowest cost generic equivalent’s list price? You read that right … as we will show in this report, Part D is collectively expending all of its “negotiating” effort on this blockbuster MS treatment to deliver far worse rates than it could get if it just purchased the lowest cost option directly from the generic manufacturer (with no rebates)! (See trailer image below if you don’t believe us)
Something doesn’t add up here. And final spoiler alert … while we have some high-conviction theories on the subject, we don’t have all of the data we need to conclusively answer why plans are hiding the truth about generic Tecfidera pricing from their members and the federal government. But we do have the data to solidly illustrate Part D’s systemic failure. So if you’re a fan of drug-pricing horror movies, please feel free to head over and begin watching our latest thriller though we don’t blame you if you just don’t have the stomach for this stuff anymore.
Thanks to Marty Schladen at the Ohio Capital Journal for his excellent coverage of the recent congressional forum on pharmacy benefit managers that we were fortunate to participate in. For more on the hearing, click here.